iPonatic III – Portable Molecular Workstation
Overview
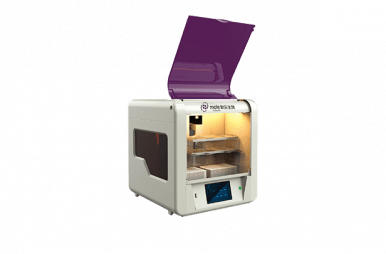
The iPonatic III – Portable Molecular Workstation is the latest innovation in Sansure’s iPonatic series, redefining molecular diagnostics with advanced capabilities. Equipped with cutting-edge technology, the iPonatic III introduces a new era of "digital and intelligent" diagnostics, empowering healthcare professionals with fast and accurate results.
Key Features
Fully automated, rapid testing process with a "sample-in, result-out" system delivering results within 15–45 minutes.
Pre-packaged reagent kits minimize hands-on time and reduce contamination risk.
An extensive and scalable testing menu addresses diverse pathogen detection needs across a wide range of scenarios.
Innovative wireless connectivity, a large smart display, and modular design enable immediate testing as samples arrive.
Lightweight at just 7.2 kg (15.84 lbs).
Enhanced with the state-of-the-art “SanUI” interactive system and HD touch screen for direct result display.
Designed to international industrial design standards, reflecting Sansure’s unique design philosophy.
Technical Specifications
Model: S-Q36A
Dimensions: 391 mm × 140 mm × 368 mm (L × W × H)
Weight: Approx. 7.2 kg
Channels: FAM, VIC/HEX, ROX/Texas Red, CY5
Test Duration: 15–45 min (e.g., SARS-CoV-2)
LOD: 200 copies/mL (SARS-CoV-2)
Max Heating Rate: ≥10℃/sec
Max Cooling Rate: ≥3℃/sec
Temperature Accuracy: ±0.5°C
Functions: Nucleic acid extraction, amplification detection, data analysis
Display: Built-in 7-inch HD touch screen, optional 12.1-inch smart screen
Interfaces/Connectivity: USB2.0, RJ45, Type-C, Wi-Fi, Bluetooth, LIS_LH7
Input Voltage: 100–240 VAC
Power Frequency: 50/60 Hz
Rated Power: 160 VA
Operating Temperature: 10°C–30°C
Storage/Transport Temperature: -40°C–55°C
Operating Humidity: 30%–80% (non-condensing)
Storage/Transport Humidity: ≤93% (non-condensing)
Barometric Pressure: 85.0–106.0 kPa
Altitude: <3,000 meters
Certification: CE
Test Menu
Respiratory Pathogens (RTI):
SARS-CoV-2 (ORF1ab, N gene)
SARS-CoV-2 (ORF1ab, N, E genes)
SARS-CoV-2/Flu A/Flu B
SARS-CoV-2/Flu/RSV
Six Respiratory Pathogens (Flu A/B, RSV, AdV, HRV, MP)
Acinetobacter baumannii and Candida albicans
Flu A/B, Mycoplasma pneumoniae, TB, Bordetella pertussis, RSV, Streptococcus pneumoniae
Carbapenemase gene (KPC), MERS, Legionella pneumophila, Adenovirus
STI & HPV Testing:
HPV 13+2 (detects HPV 16 and 18, plus pooled results for 13 other high-risk types)
HPV 15 High-Risk
HPV 16 & 18
HPV 6 & 11
HSV-2, HSV-1 & 2
MG/MH/TV
Neisseria gonorrhoeae, Ureaplasma urealyticum, Mycoplasma genitalium
CT/UU/NG combination detection
Other Infections:
Epstein-Barr Virus (EBV)
Group B Streptococcus (GBS)
Toxigenic Clostridium difficile (CD)*
Monkeypox Virus (MPXV)*